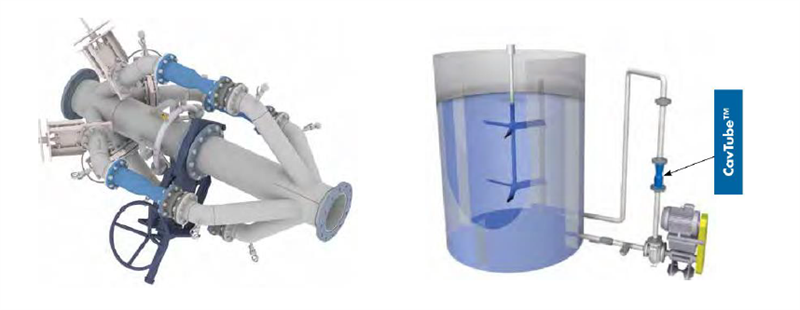
Gold Leach Tank Aeration
This was originally published by Eriez.
Gold Leaching, Gas Injection and Bubbles
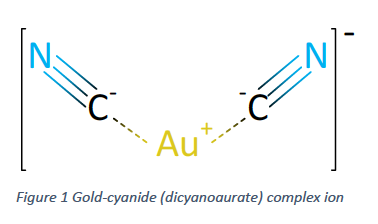
The processing of low-grade gold ores is largely made possible through the use of the cyanide leaching process. The goal of any leaching process is to selectively bring a desired mineral into solution in order to efficiently separate it from the solid waste material. While many leaching processes typically use acid or alkali solutions at high temperatures and pressures, the cyanidation process allows for gold leaching to occur at or near ambient temperature and pressure. This is possible by the formation of soluble gold-cyanide complexes (Figure 1), which is defined by the Elsner Equation. As can be seen in Equation 1, both oxygen and cyanide are required for this reaction to progress.
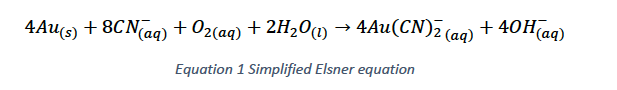
In industrial application it is typically the practice for cyanide to be used in excess, with oxygen being the rate limiting reactant. Note that in the reaction O2 is shown as aqueous; this is typically referred to as dissolved oxygen (DO). While there are several methods for increasing the DO in leaching circuits, such as gas injection and peroxide addition (hydrogen or calcium), the use of peroxide addition has tailed off significantly over the years, primarily due to the high cost. Gas injection is the most common method and is the subject of this review.
Unlike peroxide addition, gas injection requires that the oxygen first enter solution before it can participate in the reaction. The rate of dissolution of oxygen (Equation 2) is controlled by two processes: 1) mass transfer of O2 to the gas-liquid interface and 2) mass transfer of O2 into the solution through the interface. The second process being the slower of the two typically controls the rate of dissolution. The simplest way to improve this rate is by increasing the area of the gas-liquid interface. This is done by injecting (sparging) an oxygen source into the leach tank directly rather than relying on the exposed surface at the top of the tank. By injecting a gas into the leach tank bubbles are formed and the surface area of these bubbles increases the area of the gas-liquid interface.
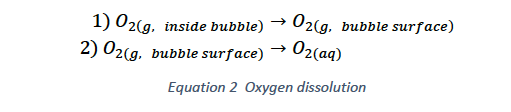
The next step to improving the oxygen dissolution rate is increasing the surface area of the bubbles. This can be done by decreasing the average bubble size, for an equivalent volume of gas. The change in surface area caused by decreasing bubble size is significant, particularly below diameters of 2 mm (see Figure 3). Smaller bubbles also have lower rise velocities and are more easily distributed within the tank, both of which increase the time a bubble spends in the solution, increasing the efficiency of the oxygen dissolution.
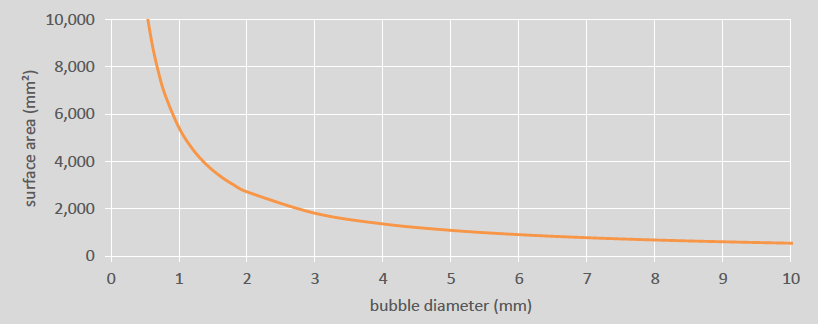
Figure 3: Surface area vs bubble size (equivalent volume)
Oxygen vs Air
As mentioned, increasing the dissolved oxygen (DO) levels is typically achieved through the introduction of gas into the leach circuit. Pre-aeration, oxidization, leaching, as well as cyanide destruct processes all employ some form of gas sparging system. The type of gas used depends on the oxygen requirements of the process. Typically, air, oxygen enriched air or high purity oxygen are used. The reason for the use of different gases is because higher DO levels that can be achieved by gases that have a higher concentration of oxygen. This relationship is governed by Henry’s law which relates the partial pressure and temperature of a gas to the amount of gas dissolved in the solution (See Figure 4). This relationship means that there is an upper limit to the DO. At some point adding more air or forming smaller bubbles will not result in an increase in DO and increasing the partial pressure of oxygen (by either enriching the air or using pure oxygen) will be necessary.
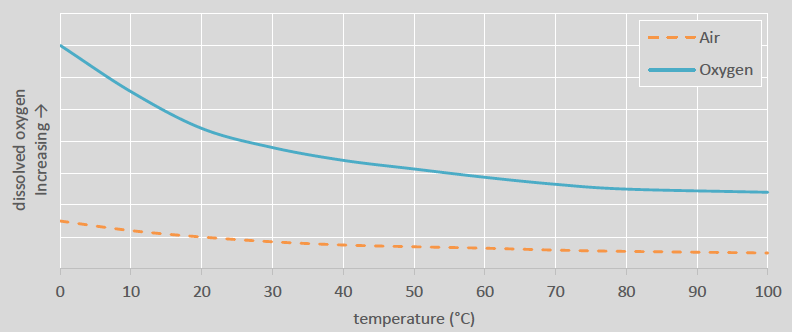
Figure 4: Maximum amount of dissolved oxygen
With this in mind, the use of air and oxygen is very common. While, the cost of oxygen is higher, the metallurgical gains realized through the use of oxygen sparging, more often than not, offset this higher cost. However, site specific considerations sometimes make air injection the preferred sparging media.
To read the full paper click here.
